Phenylacetone, sometimes abbreviated P2P is an organic compound. It is a clear oil with a refractive index of 1.5168. This chemical is used in the manufacture of methamphetamine and amphetamine. Due to the illicit uses in clandestine chemistry, it was made a controlled substance in 1979 in the United States. This substance has a very strong and persistent odor that is difficult to remove from walls, carpets, clothing, or skin. The persistence of the smell may have played a role in the decline of popularity of phenylacetone in methamphetamine synthesis and consequently in the DEA demoting phenylacetone from List I to List II.[1]
Preparation
There are many methods in the scientific literature to prepare phenylacetone, and due to its controlled nature there is crossover into popular literature such as works by Uncle Fester and Alexander Shulgin. Not surprisingly there is also a fair amount of data available on the Internet relating to the preparation of phenylacetone.
A conceptually simple example of phenylacetone organic synthesis is the Friedel-Crafts acylation of benzene with chloroacetone. This reaction has the benefit of eliminating multi-substituted products as the electron-withdrawing carbonyl-functional group deactivates the Benzene ring, eliminating multiple acylations.
Phenylacetone can also be produced from many other chemicals. For example, phenylacetic acid is distilled with lead acetate to yield phenylacetone. Another is, benzaldehyde is reacted with nitroethane yielding phenyl-2-nitropropene, which is reduced, usually in the presence of acid, to phenylacetone.
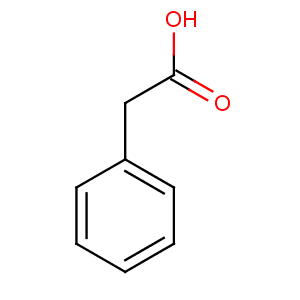
Chemicals  Phenylacetic acid Phenylacetic acid
Phenylacetic acid
CAS number [103-82-2]
IUPAC name
Phenylacetic acid
MOL WT. 136.15
H.S. CODE : 2916.33
 Identifiers Identifiers
CAS number [103-82-2]
SMILES c1ccccc1CC(=O)O
Properties
Molecular formula C8H8O2
Molar mass 136.15 g/mol
Density 1.0809 g/cm3
Melting point 76-77 °C
Boiling point 265.5 °C
Hazards
MSDS External MSDS
Phenylacetic acid (abr. PAA and synonyms are: α-toluic acid, benzeneacetic acid, alpha tolylic acid, 2-phenylacetic acid) is an organic compound containing a phenyl functional group and an acetic acid functional group.
Because it is used in the illicit production of phenylacetone (used in the manufacture of meth/amphetamines), it is subject to controls in the United States.
Physical data
Appearance: white crystals with a honey-like odour
Melting point: 77 C
Boiling point: 265 C
Vapour density:
Vapour pressure: 1 mm Hg at 97 C
Density (g/cm3): 1.228
Flash point: 190
Explosion limits:
Autoignition temperature:
Phenylacetic acid ≥ 99%
Stability
Stable. Incompatible with strong oxidizing agents.
Toxicology
Harmful by ingestion, inhalation or through skin contact. Severe eye irritant. Skin and respiratory irritant. May act as a teratogen. Oral rat LD50 2250 mg/Kg
Toxicity data
(The meaning of any abbreviations which appear in this section is given here.)
ORL-RAT LD50 2250 mg kg-1
ORL-GPG LD50 2250 mg kg-1
IPN-RAT LD50 1600 mg kg-1
Risk phrases
(The meaning of any risk phrases which appear in this section is given here.)
R20 R21 R22 R36 R37 R38.
Personal protection
Safety glasses. Adequate ventilation. NFPA RATINGS Health: 1 Flammability: 1 Reactivity: 0 FLASH POINT 190 C STABILITY Stable under ordinary conditions GENERAL DESCRIPTION & APPLICATIONS Phenylacetic acid is a white crystals with a disagreeable odor; boiling point 262 C; soluble in alcohol and ether. It serves as an ingredient in perfume to provide honey-like odor. It is found as a moiety in some alkaloids and plant hormones. It is formed as catabolite of phenylalanine. In toluic acid name system, phenylacetic acid is alpha-toluic acid which one of the hydrogen atoms in the methyl group has been substituted instead of the substitution in the benzene ring. Substituted phenylacetic acid molecule at alpha position and phenylacetate esters can serve as a drug with a wide variety of effects including anticholinergic, muscarinic antagonist, antidote to cholinesterase inhibitors or toxins, cycloplegic and mydriatic. Phenylacetic acid is used to prepare a nonsteroidal antiinflammatory drug like diclofenac. It is used the manufacture of penicillin. Mandelic Acid, phenylglycollic acid, can be produced from phenylchloracetic acid. APPEARANCE
White to creamy flaky powder PURITY (GLC) 99.0% min
MELTING POINT 76 - 78 C
WATER 1.0% max
TRANSPORTATION PACKING 25kgs in bag
HAZARD CLASS UN NO.
REMARKS Hazard Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 22/24/25
| | Note /Government Notification: These chemicals are designated as those that are used in the manufacture of the controlled substances and are important to the manufacture of the substances. For any (Control Substance) products Import and Export *** subjected to your country government laws /control substance ACT.
Information: The information on this web page is provided to help you to work safely, but it is intended to be an overview of hazards, not a replacement for a full Material Safety Data Sheet (MSDS). MSDS forms can be downloaded from the web sites of many chemical suppliers. ,also that the information on the PTCL Safety web site, where this page was hosted, has been copied onto many other sites, often without permission. If you have any doubts about the veracity of the information that you are viewing, or have any queries, please check the URL that your web browser displays for this page. If the URL begins "www.tajapi.com/www/Denatonium Benzoate.htm/" the page is maintained by the Safety Officer in Physical Chemistry at Oxford University. If not, this page is a copy made by some other person and we have no responsibility for it.
The Controlled Substances Act (CSA) was enacted into law by the Congress of the United States as Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970.[1] The CSA is the federal U.S. drug policy under which the manufacture, importation, possession, use and distribution of certain substances is regulated. The Act also served as the national implementing legislation for the Single Convention on Narcotic Drugs |  |
|
 Identifiers
Identifiers



No comments:
Post a Comment